TSCA/FIFRA/IRIS/EPCRA
NAMC Requests Extension Of TSCA Chemical Data Reporting Period/EPA Agrees: On May 18, 2012, the North American Metals Council (NAMC) was the first entity to request an extension of the reporting period for the Chemical Data Reporting (CDR) rule under the Toxic Substances Control Act (TSCA). The requested extension would result in a September 30, 2012, deadline for CDR reporting, a date that is aligned with future CDR reporting periods. According to NAMC, the extension request is necessitated by unexpected problems encountered with the electronic reporting system that the U.S. Environmental Protection Agency (EPA) requires CDR submitters to use and by EPA’s failure to respond in a timely manner to questions or requests for clarification. The additional time is essential for impacted companies to respond appropriately, adequately, and comprehensively to this complicated and time-consuming reporting requirement. On June 11, 2012, EPA signed a Federal Register notice extending the deadline for CDR submissions from June 30, 2012, to August 13, 2012. According to the pre-publication notice, this is a “one-time extension” only. The CDR regulations require manufacturers and importers of certain chemical substances included on the TSCA Inventory to report current data on the manufacturing, processing, and use of the chemical substances. The pre-publication notice is available online. More information is available online.
EPA Announces NAS Review Of IRIS Assessment Development Process: On May 16, 2012, EPA announced that the National Academy of Sciences (NAS) will conduct a comprehensive review of EPA’s Integrated Risk Information System (IRIS) program’s assessment development process. In April 2011, NAS recommended several ways to improve the development of IRIS assessments. NAS will conduct a review of the IRIS assessment development process and the changes that are currently being made or planned by EPA in response to NAS’ April 2011 recommendations. NAS will also review current methods for weight of evidence analyses and recommend approaches for weighing scientific evidence for chemical hazard identification. More information about IRIS is available online.
EPA Releases Progress Report To Congress: On June 5, 2012, EPA released the progress report that it delivered to Congress on April 20, 2012. According to EPA, the progress report provides Congress, stakeholders, and the public with an update on the IRIS Program and EPA’s progress toward implementing the NRC recommendations for improving the development of IRIS assessments. The progress report is available online.
EPA Releases Draft IRIS Ammonia Assessment: On June 8, 2012, EPA released a draft IRIS assessment for ammonia. 77 Fed. Reg. 34039. The draft assessment proposes a reference concentration (RfC) of 0.3 milligram ammonia per cubic meter of air. The RfC is an estimate of the amount of ammonia a person could inhale daily that is not likely to cause harmful health effects. The RfC proposed in the draft IRIS assessment is less stringent than the current RfC for ammonia, which is 0.1 milligram ammonia per cubic meter of air. The draft assessment covers both gaseous ammonia and ammonium hydroxide (ammonia dissolved in water). EPA estimates that approximately 80 percent of commercially produced ammonia is used as an ingredient in agricultural fertilizers. Ammonia also is used as a corrosion inhibitor, in household cleaning products, as an antimicrobial agent in food products, and as a refrigerant. A public listening session on the draft will take place on July 12, 2012. Comments are due on August 7, 2012.
EPA Extends Comment Period On PBDE Test Rule And SNUR: On May 24, 2012, EPA extended the comment period on the proposed polybrominated diphenylethers (PBDE) test rule and significant new use rule (SNUR), published on April 2, 2012. 77 Fed. Reg. 30972. The comment period was extended at the request of several entities who expressed concern in the complexity of the proposal. Comments are due by July 31, 2012.
EPA Announces Work Plan Chemicals For Assessment During 2013 and 2014: Exactly three months from the date EPA Office of Pollution Prevention and Toxics (OPPT) announced its TSCA Work Plan initiative, EPA on June 1, 2012, announced an additional 18 chemicals scheduled for assessment during 2013 and 2014. Although it is not clear from EPA’s announcement which chemicals would be assessed in which year, EPA did announce that it “would welcome the submission of additional relevant information on these chemicals, such as unpublished studies not already available through the existing literature, or information on uses and potential exposures.” That information should be submitted no later than August 31, 2012. More information is available online and online.
EPA Proposes SNUR For MCAN: On June 13, 2012, EPA proposed a SNUR under TSCA for a genetically modified microorganism identified generically as Trichoderma reesei (T. reesei). 77 Fed. Reg. 35331. This is the first SNUR EPA has issued for a microorganism, which was the subject of a Microbial Commercial Activity Notice (MCAN). EPA believes this action is necessary because the use of this genetically modified T. reesei under certain conditions may be hazardous to human health and the environment. This proposed rule would also establish a mechanism to allow EPA to evaluate an intended use and its conditions, and to prohibit or limit that activity before it occurs, if EPA determines it may be hazardous. Comments must be received on or before July 13, 2012.
EPA Issues SNUR On Elemental Mercury Used In Barometers, Manometers, Hygrometers, And Psychrometers: On May 30, 2012, EPA issued a final SNUR under TSCA for elemental mercury use in barometers, manometers, hygrometers, and psychrometers. 77 Fed. Reg. 31728. This rule requires persons who intend to manufacture (including import) or process elemental mercury for an activity that is designated as a significant new use by this final rule to notify EPA at least 90 days before commencing that activity. The required notification will provide EPA with the opportunity to evaluate the intended use and, if necessary, to prohibit or limit that activity before it occurs. Use of elemental mercury in these articles that were in service prior to May 6, 2011, will not be covered as a significant new use under this SNUR. Also, use of mercury in portable, battery-powered, motor-aspirated psychrometers that contain fewer than seven grams of mercury is an ongoing use and therefore will not be covered by this SNUR. This final rule is effective June 29, 2012.
ITC Issues Report To EPA: On May 23, 2012, EPA announced that the TSCA Interagency Testing Committee (ITC) transmitted its 69th Report to the EPA Administrator on April 5, 2012. 77 Fed. Reg. 30856. In the 69th ITC Report, the ITC is adding a category of cadmium compounds, including any chemical that contains cadmium as part of that chemical’s structure, six non-phthalate plasticizers, 25 phosphate ester flame retardants, two other flame retardants, nine chemicals to which children living near hazardous waste sites may be exposed, and a category of 69 diisocyanates and related compounds (including 14 Action Plan chemicals and 55 related compounds) to the TSCA Priority Testing List. In addition, the ITC is removing 103 cadmium compounds and 14 High Production Volume (HPV) Challenge Program orphan chemicals from the Priority Testing List during this reporting period (June to November 2011). The ITC is adding the category of cadmium compounds and removing 103 cadmium compounds to provide a more comprehensive approach to assessing cadmium compounds’ safety. Comments must be received on or before June 22, 2012. On June 11, 2012, EPA announced that the ITC forwarded its 70th Report to the Administrator of EPA. 77 Fed. Reg. 34778. No changes to the Priority Testing List were recommended.
EPA Hosts Meeting On Chemical Data Submissions: EPA hosted a meeting on May 31, 2012, with industry and nongovernmental organizations (NGO) interested in EPA’s public release of information it is gathering under the CDR rule. Among the questions EPA posed, is what types of electronic search capabilities EPA should provide to help interested parties review the information. EPA also asked for opinions about which types of data analyses should be submitted in the near term and what types in the long term.
OPP Announces Personnel Changes: On May 31, 2102, EPA announced that Dr. Karen Whitby, Acting Director of the Health Effects Division (HED), will retire from federal service at the end of June. Whitby had more than 30 years of federal service, 20 of which have been with the Office of Pesticide Programs (OPP), including as a Senior Advisor in the Immediate Office where she led a team to help implement the Endocrine Disruptor Screening Program. Jack Housenger will become Director of HED at the end of June. Housenger is currently the Director of the Biological and Economic Analysis Division (BEAD) where he has managed a Division consisting of scientists, economists, and regulatory experts, including staff in three laboratories located in Maryland and Mississippi. Housenger has been Associate Director of HED, Associate Director of the Antimicrobials Division, and Associate Director of the former Special Review and Reregistration Division. Susan Lewis, Associate Division Director for BEAD, was named Acting Director.
Ken Olden Named Director Of National Center For Environmental Assessment: On May 31, 2012, Dr. Kenneth Olden was named head of the National Center for Environmental Assessment (NCEA) and the Human Health Risk Assessment Research Program. Olden was Director of the National Institute of Environmental Health Sciences (NIEHS) and the National Toxicology Program (NTP) in the U.S. Department of Health and Human Services from 1991-2005. Olden is currently the Founding Dean of the School of Public Health at the Hunter College, City University of New York. Olden received his Bachelor of Science in biology from Knoxville College, a Master of Science in genetics from the University of Michigan, and a Doctorate in cell biology and biochemistry from Temple University. Olden will join EPA on July 2, 2012.
EPA Sued Over Lead Bullets: On June 7, 2012, a coalition of seven environmental organizations, including the Center for Biological Diversity and the Preserve Our Wildlife Organization, filed suit against EPA for failure to act on a March petition related to lead ammunition. Trumpeter Swan Society v. EPA, D.D.C., docket number not available (June 7, 2012). The complaint seeks declaratory and injunctive relief and requests the court to order EPA to develop and implement regulations to “adequately protect wildlife, human health, and the environment” from lead ammunition used in hunting and shooting sports. The plaintiffs assert that EPA’s decision to regard the March petition, which requested EPA initiate a rulemaking pursuant to TSCA Section 21, as “substantially the same” as an August 2010 petition to be a TSCA violation. The complaint states EPA has the authority to regulate lead in ammunition and the petition “clearly demonstrates” the need for such regulation to reduce the potential of harmful lead exposure to wildlife and humans.
State FIFRA Issues Research And Evaluation Group Meeting: On May 30-31, 2012, the Association of American Pesticide Control Officials (AAPCO)/State FIFRA Issues Research and Evaluation Group (SFIREG) Pesticide Operations Management (POM) Working Committee (WC) held its semi-annual full committee meeting in EPA Region 5 offices in Chicago, Illinois. Through a cooperative agreement in 1978, EPA and AAPCO created SFIREG, an AAPCO committee with EPA funding, to promote information exchange and cooperation between the states and EPA in the development of pesticide policies and regulations. More information about SFIREG and its committees is available online. A copy of the meeting agenda is available online. More information about the meeting is available online.
CAA/CWA
NGOs Urge Use Of CAA Section 112(r) To Achieve Inherently Safer Technology: In a May 16, 2012, letter to President Obama, environmental, labor, and public health organizations asked the President to use Clean Air Act (CAA) Section 112(r) to ensure that high-risk chemical plants prevent releases of hazardous chemicals in the event of a terrorist attack. Since passage of the 2006 Department of Homeland Security’s Chemical Facility Anti-Terrorism Standards, many of these groups have urged Congress to include an “inherently safer technology” provision in chemical security legislation. The provision was included in H.R. 2868, a bill that passed the House in 2009 but died in the Senate. According to the NGOs, “ntil Congress acts responsibly, the only way to ensure communities are protected from chemical disasters is to fully enforce the 1990 Clean Air Act.” The Act’s “general duty clause” under Section 112(r) requires “all chemical facilities to be designed and operated to prevent catastrophic chemical releases.” Greenpeace and Public Citizen’s Litigation Group prepared a memorandum to senior staff within EPA’s Office of Solid Waste and Emergency Response and Office of Air and Radiation, outlining their analysis of EPA’s authority for requiring inherently safer technology at chemical plants. The memorandum pointed to EPA’s authority under the CAA Section 112(r)’s general duty clause. It also referred to EPA’s unused authority under the Act “to promulgate release prevention, detection, and correction requirements which may include monitoring, record-keeping, reporting, training, vapor recovery, secondary containment, and other design, equipment, work practice, and operational requirements.” The letter sent to President Obama by the coalition of groups is available online. The memorandum by Greenpeace and Public Citizen’s Litigation Group is available online.
RCRA/CERCLA DEVELOPMENTS
OIG Criticizes EPA “Inaction” On Pharmaceutical Waste: On May 25, 2012, the Office of Inspector General (OIG) issued a report criticizing EPA for its “inaction” in addressing hazardous waste pharmaceutical disposal. The report, EPA Inaction in Identifying Hazardous Waste Pharmaceuticals May Result in Unsafe Disposal, urges EPA to identify and review existing pharmaceuticals to see if any qualify as hazardous waste, establish a procedure for reviewing new pharmaceuticals to see if they qualify as hazardous waste where disposed, and conduct a national outreach and compliance program to ensure hazardous waste pharmaceuticals are disposed of properly. EPA has the authority under the Resource Conservation and Recovery Act (RCRA) to determine whether pharmaceuticals should be regulated as hazardous waste. EPA has not updated the list of hazardous waste pharmaceuticals in over 30 years. EPA proposed amendments to its universal waste rule for regulating hazardous waste pharmaceuticals (HWP) in 2008, but EPA abandoned it. EPA reportedly intends to offer a new proposal for regulating hazardous waste pharmaceuticals by spring 2013. OIG’s report is available online.
GREEN CHEMISTRY DEVELOPMENTS
California Issues Revised Informal Draft Safer Consumer Products Regulations: At the request of California Governor Jerry Brown (D), the California Department of Toxic Substances Control (DTSC) withdrew its October 31, 2011, informal draft Safer Consumer Products regulations, as well as a revised formal draft scheduled for release in April. Brown reportedly had concerns regarding the scope of the regulations, the treatment of confidential business information, and duplication of existing federal and state regulations. Brown asked DTSC to issue new informal draft regulations for a limited one-week comment period. The revised informal draft regulations, dated May 18, 2012, are available online. More information is available online.
NANOTECHNOLOGY
International Symposium Slides And Videos Of The Plenary Presentations Are Now Available: On March 27-28, 2012, the Organization for Economic Cooperation and Development (OECD), in collaboration with the National Nanotechnology Initiative (NNI), and hosted by the American Association for the Advancement of Science (AAAS), held an International Symposium on Assessing the Economic Impact of Nanotechnology. The objective of the symposium was to explore systematically the need for and development of a methodology to assess the economic impact of nanotechnology across whole economies, factoring in many sectors and types of impact, including new and replacement products and materials, markets for raw materials, intermediate and final goods, and employment and other economic impacts. Lynn L. Bergeson was on the Steering Committee and presented at the symposium. The presentation slides and plenary videos are now available online.
EU Biocides Regulation Addresses Nanomaterials: On May 10, 2012, the Council of the European Union (EU) announced the adoption of a regulation concerning the placing on the market and use of biocidal products, which include insecticides, disinfectants, and repellents, but not medicines or agricultural pesticides. The regulation will take effect September 1, 2013, with a transitional period for certain provisions. The regulation incorporates the European Commission’s (EC) recommendation on the definition of a nanomaterial, and requires that, where nanomaterials are used in a product, the risk to the environment and to health be assessed separately. Labels would be required to include the name of all nanomaterials contained in biocidal products, followed by the word “nano” in brackets. The regulation states that “approval of an active substance shall not cover nanomaterials except where explicitly mentioned.” More information is available online.
Health Council Of The Netherlands Proposes Registry For Worker Exposure To Engineered Nanoparticles: The Health Council of the Netherlands announced on May 22, 2012, the availability of a draft report proposing the implementation of an exposure registry and a system of health monitoring when working with engineered nanoparticles. The draft report states that, due to the concerns and lack of knowledge, the Health Council “considers it prudent” to create an exposure registry. The Health Council recommends that the exposure registry be created for “insoluble and poorly in water soluble nanoparticles in any composition or physical structure, including nanoparticles that are present in solid materials.” The draft report acknowledges that, if solid materials are in good condition, “scarcely any nanoparticles will be released, but due to wear and tear and handling, such as drilling and sanding, it cannot be excluded that such particles can be released with all the associated risks.” The draft report concludes that, “[f]rom the point of view of health, it is best to also register the solid materials.” Data submitted to the registry would need to include chemical and physical properties, determinants of emission and exposure, and exposure concentrations. Regarding medical surveillance, the draft report concludes that implementation of a passive system is the best option. While a passive system would not provide answers quickly on whether health risks exist when working with nanoparticles, and if so, which type of health effects, when combined with other activities, such as targeted scientific research, it “may give a valuable contribution in the future to providing insight in the potential health risks due to exposure to nanoparticles.” Comments on the draft report, which was presented to experts of employer’s organizations and trade unions, are due August 10, 2012. According to the Health Council, it will consider comments when preparing the final report, which will be presented to the State Secretary of Social Affairs and Employment. More information is available online.
ECHA Updates Additional Guidance For Nanomaterials: On May 25, 2012, the European Chemicals Agency (ECHA) published three new appendices, updating Chapters R.8, R.10, and R.14 of the Guidance on Information Requirements and Chemical Safety Assessment. ECHA updated the guidance based on the outcome of the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) Implementation Projects on Nanomaterials (RIP-oN) 3, which concerned aspects such as occupational exposure estimation and dose-response characterization for human health and for environment. According to ECHA, the updated appendices “will further enhance the advice to registrants of substances in the nanoform a full year in advance of the next REACH registration deadline,” May 31, 2013. More information is available online.
ISO Publishes Technical Report On Physical Characterization Of Engineered Nanomaterials: The International Organization for Standardization (ISO) has published a Technical Report (TR) providing guidance on the physicochemical characterization of manufactured nano-objects prior to toxicological assessment. ISO states that TR 13014:2012, Nanotechnologies — Guidance on physicochemical characterization of engineered nanoscale materials for toxicologic assessment, “is intended to assist health scientists and experts to understand, plan, identify, and address relevant physicochemical characteristics of nano-objects before conducting toxicological tests on them.” ISO Technical Committee (TC) 229 Working Group (WG) 3, Health, Safety, and Environmental Aspects of Nanotechnologies, prepared the TR. The U.S. Technical Advisory Group (TAG) to ISO TC 229, which is accredited and administered by the American National Standards Institute (ANSI), is open to all materially affected U.S. national interested parties. The U.S. TAG formulates positions and proposals on behalf of the U.S. with response to ISO standardization activities, and provides the delegates and experts who represent the U.S. at meetings of the respective ISO TCs, subcommittees, and WGs. The TR is available online. More information concerning the U.S. TAG is available online.
NIOSH Releases Guidance On General Safe Practices For Working With Engineered Nanomaterials In Research Laboratories: The National Institute for Occupational Safety and Health (NIOSH) has posted a document entitled General Safe Practices for Working with Engineered Nanomaterials in Research Laboratories, which contains recommendations on engineering controls and safe practices for handling engineered nanomaterials in laboratories and some pilot scale operations. According to NIOSH, it designed the guidance “to be used in tandem with well-established practices and the laboratory’s chemical hygiene plan.” The guidance notes that experimental animal studies indicate that potentially adverse health effects may result from exposure to nanomaterials, and that the routes of exposure include inhalation, dermal exposure, and ingestion. The guidance concludes that “[t]he full range of occupational hygiene controls will be necessary to limit exposures to nanomaterials as a means to prevent adverse health outcomes in the research community. Engineering and administrative controls can eliminate or minimize the amount of nanomaterials that will be present in workplace air or settled on surfaces. Personal protective equipment can be used where other types of controls are not available or practical.” The guidance is available online.
ECHA Will Create Working Group On Nanomaterials: ECHA recently held a two-day workshop concerning its first experiences with nanomaterials under REACH, with an emphasis on the evaluation process. ECHA, Member State Competent Authorities (MSCA), accredited stakeholders, and the EC discussed how nanomaterials in general have been characterized in registration dossiers. Currently, according to ECHA, the scope of the registration (i.e., whether and how many nano-forms are included) is often unclear and the level of nano-specific information provided (e.g., substance characterization, hazards, exposure, and risks) shows “significant room for improvement.” ECHA agreed with MSCA representatives on a common approach to addressing the current information requirements in nanomaterial dossiers, taking into account the scientific uncertainties and legislative framework provided by REACH. ECHA states that it will implement the EC’s recommendation on the definition of a nanomaterial as a benchmark in assessing substances, and “invites registrants to proactively characterise their substances in light of this definition.” Workshop participants discussed creating a working group on nanomaterials that would provide advice on scientific and technical principles related to nanomaterials under REACH. The working group on nanomaterials would act independently, but report to the relevant ECHA committees. According to ECHA, the mandate of this working group will be further consolidated with the MSCAs. In addition, ECHA intends to disseminate the best practices that it has collected from relevant stakeholders that registered nanomaterials and that were discussed in the first “Group Assessing Already Registered Nanomaterials” meeting prior to the workshop. ECHA intends to post the best practices on its website by this summer. More information is available online.
U.S. Delegation May Present Nanotechnology Guidance At UN GHS Subcommittee Meeting: The U.S. delegation to the July 4-6, 2012, meeting of the United Nations (UN) Subcommittee of Experts on the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) is considering presenting an information paper on how to classify nanomaterials under the GHS. According to Kathy Landkrohn, Occupational Safety and Health Administration (OSHA) Directorate of Standards and Guidance, the paper would be presented under a work group examining the types of physical and chemical properties listed on safety data sheets (SDS). Landkrohn stated that a lack of data has impeded the work group’s ability to create a separate hazard class for nanomaterials.
OSHA
OSHA GHS Rule – Petition for Review: On March 26, 2012, OSHA issued in final its Hazard Communication Rule to amend its Hazard Communication Standard to be consistent with the UN’s GHS. On May 23, 2012, CropLife America, American Petroleum Institute, American Chemistry Council, American Tort Reform Association, the National Grain and Feed Association, and multiple other trade associations filed Petitions for Judicial Review of the final rule. According to American Petroleum Institute, two significant issues are of concern to its membership: OSHA’s treatment of petroleum streams as “complex mixtures” instead of “complex substances”; and the fact that in the Notice of Proposed Rulemaking combustible dust was listed under Unclassified Hazards (Hazards Not Otherwise Classified). All of industry’s combustible dust comments were directed at this approach. Because industry was unable to comment on the position taken in the final rule where combustible dust is listed separately as a hazardous chemical with specified label elements, the challenge was needed. CropLife America is concerned with potential conflicts between pesticide labels use under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and GHS requirements.
Federal Advisory Council On Occupational Safety And Health Considers PEL Recommendations: The Federal Advisory Council on Occupational Safety and Health (FACOSH) met on May 3, 2012, during which it discussed revised recommendations on permissible exposure limits (PEL) from FACOSH’s Emerging Issues Subcommittee. FACOSH is authorized by the Occupational Safety and Health Act and Executive Order (EO) 11,612, as amended, “to advise the Secretary [of Labor] on all matters relating to the occupational safety and health of federal employees,” including providing advice on how to reduce the number of injuries in the federal workforce and “how to encourage each Federal Executive Branch department and agency to establish and maintain effective occupational safety and health programs.” Materials in the docket, which is available online, include a revised document entitled “Recommendations for Consideration by the U.S. Secretary of Labor on the Adoption and Use of Occupational Exposure Limits by Federal Agencies” (Recommendations), available online. More information is available online.
REACH
ECHA Adds Chemicals To SVHC List: On June 12, 2012, ECHA reported that it will add chemicals to the candidate list (CL) of chemicals that could be banned in the EU under REACH. The addition to the list means the chemicals are considered “substances of very high concern” (SVHC) under REACH. The chemicals to be added to the CL are diboron trioxide, C.I. Basic Violet 3, C.I. Basic Blue 26, C.I. Solvent Blue 4, and 4,4′-bis(dimethylamino)-4″-(methylamino)tri tyl alcohol, along with eight other substances that ECHA declined to name for administrative reasons. There are now 73 substances on the CL. The total after the addition of the 13 new substances will only be 84 because in two cases substance entries would be merged. More information is available online.
LEGISLATIVE DEVELOPMENTS
Food And Drug Administration (FDA) Reform Bills Move Through Congress: On May 15, 2012, Senator Tom Harkin (D-IA) introduced S. 3187, the Food and Drug Administration Safety and Innovation Act. The measure passed the Senate by the bipartisan margin of 96-1 on May 24. The House acted promptly as well. On May 9, Representative Fred Upton (R.MI.) had introduced H.R. 5651, the Food and Drug Administration Reform Act of 2012, a bill similar to the Senate bill, but with a number of differences. On May 30, on a Motion to Suspend the Rules, the House passed the measure by a significant bipartisan margin of 387-5. The next step will be a Senate-House conference to reconcile differences between the two bills. Reports indicate that the leaders in Congress hope to have the differences resolved and a bill passed by early July. The legislation is intended to reauthorize existing user fees and the extension of such fees to new regulated products. Prescription drug application fees were reenacted, as were prescription drug establishment and product fees. Device fees were passed for 510(k)s (premarket notifications), Premarket Approval Applications (PMA), and various other related applications. Establishment fees were extended, and the definition of who has to register revised to include approximately 6,000 additional medical device firms. Companies will want to review the text as finally agreed to by the conferees to determine if the establishment definition as proposed, and eventually passed, affects them. User fees for generic drugs are included in the two bills. There are three fees involved, one for drug master files for Active Pharmaceutical Ingredients (API), one for filed Abbreviated New Drug Applications (ANDA), and one for facilities where generic drugs, APIs, and generics with APIs are produced. In a move to tighten controls, a new provision has been added to the Federal Food, Drug, and Cosmetic Act (FFDCA) providing that a drug, API, or drug with an API is misbranded if the fees have not been paid and other required information has not been provided to the agency. For biosimiliar biological products, there are provisions for three stages of fees: development programs, product applications, and establishment fees. Steps were also taken to permanently authorize the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act, and provide for instances of exclusivity for companies fostering work on rare pediatric diseases. The Humanitarian Device Exemption would also be extended.
Medical devices would be regulated differently. The changes address revisions to the classification scheme, post-market studies and surveillance, the reporting of adverse effects (devices to be made part of the drug Sentinel system), and the institution of a full blown recall scheme, with requirements for risk analysis and mitigation measures. The House and Senate bills both include provisions requiring that the Center for Devices and Radiological Health (CDRH) provide substantive, written summaries of the scientific and regulatory rationale for the denial of various PMA, 510(k), and Investigational Device Exemptions (IDE). Applicants would have the right to seek supervisory review of any such denials on request. Other provisions deal with good guidance practices for devices.
There are a variety of provisions intended to increase information about facilities where excipients are produced, about imports, as well as provisions dealing with foreign inspections. Several provisions deal with such topics as incentives for anti-infective drugs, drug shortages, medical gas regulation, and various administrative provisions.
As stated above, the final language of the FDA Reform measure will not be available until the conference committee does its work, reportedly before the Fourth of July recess.
Patient Medication Information: On May 22, 2012, Senator Kirstin Gillibrand (D-NY) introduced the Cody Miller Initiative for Safer Prescriptions Act. The bill would require the Secretary of Health and Human Services to promulgate regulations within two years after passage regarding the authorship, content, format, and dissemination of Patient Medication Information (PMI) to ensure patients receive consistent and high-quality information about their prescription medications and are aware of the potential risks and benefits of prescription medications. The regulations would have to require that the PMI be scientifically accurate and based on the professional labeling approved by the Secretary and authoritative, peer-reviewed literature, including nontechnical, understandable, plain language that is not promotional in tone or content. The measure mandates the information to be in the PMI, including the established name of the drug, drug uses, and clinical benefits; general directions for proper use; contraindications, common side effects, and most serious risks of the drug, especially with respect to certain groups such as children, pregnant women, and the elderly; measures patients may be able to take, if any, to reduce the side effects and risks of the drug; and when a patient should contact his or her health care professional.
Gray Market: On May 22, 2012, Representative Elijah Cummings (D-MD) introduced H.R 5853, the Gray Market Drug Reform and Transparency Act of 2012. The reform measure would prohibit wholesale distributors of prescription drugs from purchasing such drugs from pharmacies, except in limited circumstances. Wholesale distributors would also have to report information about their locations and officers to FDA on an annual basis, pay fees, and identify sale prices the distributor paid pharmacies for drugs listed on the FDA website as being subject to shortages. This last proviso resulted in part from an investigation by Representative Cummings into speculation by “gray market” drug companies that trade in drugs that are in critically short supply, according to FDA. The drugs in question treat life threatening illnesses such as leukemia in children, breast cancer, and seizures. As part of this investigation, Representative Cummings obtained confidential information relating to companies that charge prices many times higher than those negotiated with authorized manufacturers and distributors.
Oil, Gas, Natural Gas, And EPA: Representative Ed Whitfield (R-KY) and others introduced H.R. 4471, a bill to require analyses of the cumulative impacts of certain rules and actions of the EPA that impact gasoline, diesel fuel, and natural gas prices, jobs, and the economy, and for other purposes. The bill passed out of the House Energy and Commerce Committee and is expected to reach the House floor by the end of June. The bill requires the President to establish the Transportation Fuels Regulatory Committee to analyze and report on the cumulative impacts of certain rules and actions of EPA on gasoline, diesel fuel, and natural gas prices. The Committee is to be composed of a number of Cabinet members, the Administrator of EPA, or their designees, the Chairman of the United States International Trade Commission, acting through the Director of the Office of Economics, and the Administrator of the Energy Information Administration. The Committee is to analyze the impact of certain covered rules and actions listed in the bill to determine their impact on changes in gas, diesel, and natural gas prices, required capital investment, global competitiveness of the U.S. and other cumulative costs and cumulative benefits, and state and regional employment, including impacts associated with changes in gasoline, diesel fuel, or natural gas prices and facility closures. The bill provides for preliminary and final reports, and includes a Section 5 that prohibits the EPA Administrator from finalizing any of three listed rules until a date (to be determined by the Administrator) that is at least six months after the day on which the Committee submits the final report. The three rules are: (1) Control of Air Pollution From New Motor Vehicles: Tier 3 Motor Vehicle Emission and Fuel Standards, as described in the Unified Agenda of Federal Regulatory and Deregulatory Actions under Regulatory Identification Number 2060-AQ86, and any successor or substantially similar rule; (2) any rule proposed after March 15, 2012, establishing or revising a standard of performance or emission standard under CAA Sections 111 or 112 that is applicable to petroleum refineries; and (3) any rule revising or supplementing the national ambient air quality standards for ozone under CAA Section 109. Finally, in revising or supplementing any national primary or secondary ambient air quality standards (NAAQS) for ozone under CAA Section 109, the EPA Administrator is directed to take into consideration feasibility and cost.
No Clean Water Act Guidances: The House Transportation and Infrastructure Committee reported out H.R. 4965, a bill “[t]o preserve existing rights and responsibilities with respect to waters of the United States, and for other purposes.” The bill provides that the Secretary of the Army and the Administrator of EPA are prohibited from (1) finalizing, adopting, implementing, administering, or enforcing the proposed guidance described in the notice of availability and request for comments entitled “EPA and Army Corps of Engineers Guidance Regarding Identification of Waters Protected by the Clean Water Act” (EPA-HQ-OW-2011-0409) (76 Fed. Reg. 24479 (May 2, 2011)); and (2) using the guidance described in paragraph (1), or any substantially similar guidance, as the basis for any decision regarding the scope of the Federal Water Pollution Control Act or any rulemaking. H.R. 4965 makes it clear that the use of the guidance described in the bill, or any substantially similar guidance, as the basis for any rule would be grounds for vacating the rule. The legislation was prompted by what Republicans called the EPA effort to expand federal regulatory jurisdiction over a variety of water bodies.
RAPID Reported Out Of Committee: On June 6, 2012, by a vote of 14 to 8, the House Judiciary Committee ordered to be reported the Responsibly and Professionally Invigorating Development Act of 2012 (RAPID). According to the Declaration of Purpose, RAPID would establish a framework and procedures to streamline, increase the efficiency of, and enhance coordination of, agency administration of the regulatory review, environmental decision-making, and permitting process for projects undertaken, reviewed, or funded by federal agencies. RAPID is said to ensure that agencies administer the regulatory process in a manner that is efficient so that citizens are not burdened with regulatory excuses and time delays. RAPID would achieve these objectives by making changes to the process whereby environmental impact statements are developed and brought to fruition. A firm two-year deadline would be established for a record of decision for and Environmental Impact Statement, and one year for an Environmental Assessment Statement, the latter if the lead agency on a project determines that there is no significant environmental impact. RAPID would apply only to construction projects undertaken with federal funds. The fate of RAPID is not certain. Democrats in Congress strongly oppose the measure, as does the White House.
Bill Extends CFATS Program: The current Chemical Facility Anti-Terrorism Standards (CFATS) Program has been extended by the House through October 4, 2013, as part of the appropriations bill for the Department of Homeland Security. A Senate measure is awaiting floor action after being reported out by the Appropriations Committee. Chemical Security Action reports on its website that the Senate bill includes $86.5 million for CFATS, while the House bill includes $40 million less.
MISCELLANEOUS
OEHHA Proposes MADL For Chloroform: On May 18, 2012, the Office of Environmental Health Hazard Assessment (OEHHA) proposed to adopt a Proposition 65 Maximum Allowable Dose Level (MADL) of 660 micrograms per day for inhalation exposures to chloroform. Proposition 65 prohibits a person in the course of doing business from knowingly and intentionally exposing any individual to a chemical that has been listed as known to the State to cause cancer or reproductive toxicity, without first giving clear and reasonable warning to such individual. The Act also prohibits a business from knowingly discharging a listed chemical into water or onto or into land where such chemical passes or probably will pass into any source of drinking water. The proposed regulation would adopt the following MADL for chloroform, by amending Section 25805 as follows (addition in underline):
(b) Chemical Name
Chloroform
Level(Micrograms/day)
660 (inhalation)
Comments are due by July 2, 2012.
CDC Advisory Committee Makes Childhood Level Poisoning Recommendations: On May 17, 2012, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) recommended that the term “level of concern” be eliminated from all future agency policies, guidance documents, and other CDC publications, and that current recommendations based on the “level of concern” be updated. The ACCLPP also recommended that the CDC should use a childhood blood lead level (BLL) reference value based on the 97.5th percentile of the population BLL in children aged 1-5 years (currently 5 microorganism of lead per deciliter µg/dL) to identify children living or staying for long periods in environments that expose them to lead hazards. Additionally, the reference value should be updated by CDC every four years based on the most recent population-based-blood-level surveys conducted among children. These recommendations effectively increase, according to CDC, the number of children with elevated BLL from 250,000 to 450,000. Historically, CDC has considered children ages 1-5 who have 10 µg/dL or more of blood in their system to have “levels of concern.” This level of concern has been required with a BLL that is in the top 2.5 percent of the population. The value will be revisited every four years. The recommendations that CDC’s ACCLPP made in January, CDC’s response, and a related fact sheet are available online.
Australia Announces 3,000 Chemicals To Be Assessed In IMAP Framework: On June 15, 2012, the National Industrial Chemicals Notification and Assessment Scheme (NICNAS) announced the 3,000 existing chemicals that it will assess through its new Inventory Multi-tiered Assessment and Prioritization (IMAP) framework. NICNAS has designated the chemicals in the first group to be assessed as “Stage One chemicals.” NICNAS identified the Stage One chemicals based on characteristics agreed by stakeholders as priorities for early consideration and sources subsequently identified by NICNAS and stakeholders. These characteristics and sources of information include:
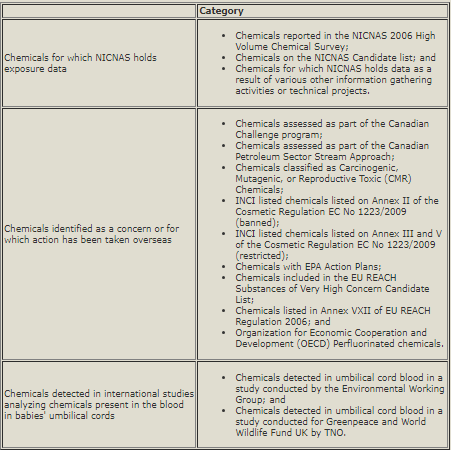
NICNAS states that the IMAP framework is a science- and risk-based model designed to align the assessment effort with the human health and environmental impacts of chemicals. It consists of three levels (tiers) of assessment, with the assessment effort increasing with each tier. Assessment outcomes from Stage One will determine whether a chemical:
Poses no unreasonable risk to human health or the environment; or
Requires risk management measures to be instituted for safe use; or
Requires more in-depth assessment to determine fully its impact on human health and/or the environment.
According to NICNAS, it will screen and assess Stage One chemicals over the next four years, up to at least Tier II. It intends to publish the outcomes (including recommendations for risk management measures) in several batches on the NICNAS website. NICNAS has identified 800 chemicals for assessment in 2012-13, and “welcomes the provision of information by introducers and users of the Stage One chemicals that will be assessed in the first year.” Information is due September 15, 2012. More information is available onilne.
This Update is provided as a complimentary service to our clients and is for informational purposes. This Update may be copied or quoted, provided proper attribution is given. The contents are not intended and cannot be considered as legal advice.
